N Winkleman1, DVM; G Silva2, DVM, PhD; P Smith3, Pharm D, MBA; C Pueggel3, BS; B Payne3, DVM
1Swine Services Unlimited, Inc., Rice, MN; 2Iowa State University, Ames, IA; 3Veterinary Pharmaceutical Solutions, St. Peter, MN
Introduction
Streptococcus suis (Ss) is a leading diagnosis at US laboratories.1 Practitioners prescribe individual (injectable) and population (oral) treatments. Antibiotic choice through AMDUCA decision-making and drug dosages have impacts on therapy success. This study objective was to provide clinical sign and production data comparisons between oral extra-label penicillin and compounded amoxicillin at various rates.
Materials and Methods
- PRRSV/PED-negative barrows (n=120) were weaned (day -8) into a BSL-2 facility
- Allocated into 6 treatment groups by weight (Table 1)
- Pigs were housed by treatment (4 pens of 5 pigs) with pen-specific feeders and drinkers.
| Table 1. Trial treatment groups | ||||
| Group # | Treatment name | S. suis challenge | Antiboitic administration for 7 days treatment | # pigs and pens |
| T1 | Challenge control | Yes | none | 20 pigs/tx 4 pens/tx 5 pigs/pen |
| T2 | Amoxicillin Red 37.5 | Yes | 37.5g/gal stock solution | |
| T3 | Amoxicillin Red 60 | Yes | 60g/gal stock solution | |
| T4 | Amoxicillin Red 120 | Yes | 120g/gal stock solution | |
| T5 | Penicillin G Potassium | Yes | 0.5BU/2.5gal stock solution | |
| T6 | Strict Negative control | No | None | |
- Pigs acclimated for 7 days before treatments were started (d-1) and challenged (d0). (Figure 1)
- T1-T5 were challenged with Streptococcus suis-type 1 (2 ml intramuscular, 2 ml intraperitoneal)
- T2-T5 received their daily-prepared, seven-day oral medications on day -1 – day 6. T2-T4 treatments were prepared as 1:128 dilutions of 37.5, 60 and 120 grams compounded VPS Amoxicillin Red/gallon of stock solution (VPS, St. Peter, MN), respectively. T5 treatment was a 1:128 dilution of 0.5BU Penicillin G Potassium/2.5 gallons of stock solution (R-Pen®, Huvepharma, Peachtree City, GA)
- T6 was housed in a different airspace to evaluate for any vertical Ss infection
Figure 1: Trial timeline including treatment, challenge, weigh dates

- Individual clinical sign severities (0-4) were recorded (days 0-10, 13, 15, 21, 23, 28, 31, 38, 42).
- Mortalities were necropsied and recorded.
- Pigs were weighed on days -8, 0, 10, 21, 42.
- No individual treatments were administered.
- Euthanasia was performed for welfare reasons following IACUC protocol.
- Regression models were used to assess intergroup ADG and mortality differences. Chi-square was used to assess clinical sign differences.
Results
- Clinical signs (Chart 1), mortality (Chart 2), ADG (Chart 3), necropsy findings, lab submissions (n=28 pigs) results confirmed successful challenge.
- T2-T6 had statistically higher ADG compared to T1. T2-T4 and T6 had >0.5lb/day higher ADG over T5 with P=0.08, P=0.14, P=0.07 and P=0.18, respectively. (Chart 3)
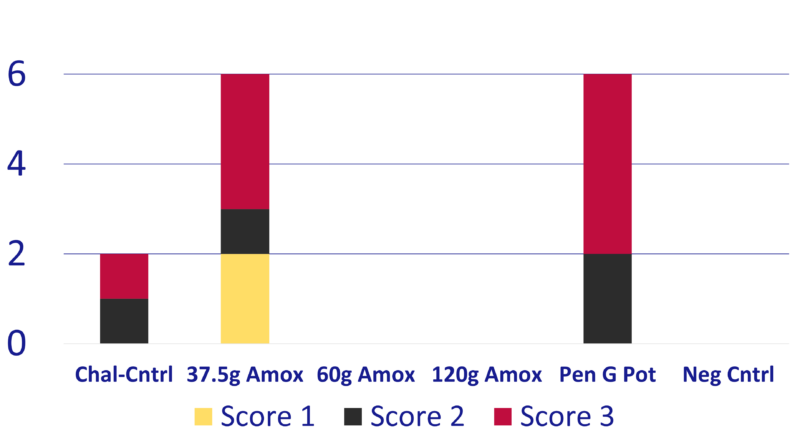
- Of the trial survivors, only T1 and T5 had pigs with clinical signs. All T3, T4, and T6 survivors had no clinical signs throughout the study. (Chart 4)
- Molecular Ss isolates’ capsular regions and 16s genomic analysis were 99.9-100% identical to the challenge isolate. Isolates were penicillin and ampicillin sensitive.
Chart 1: Percentage pigs with clinical signs
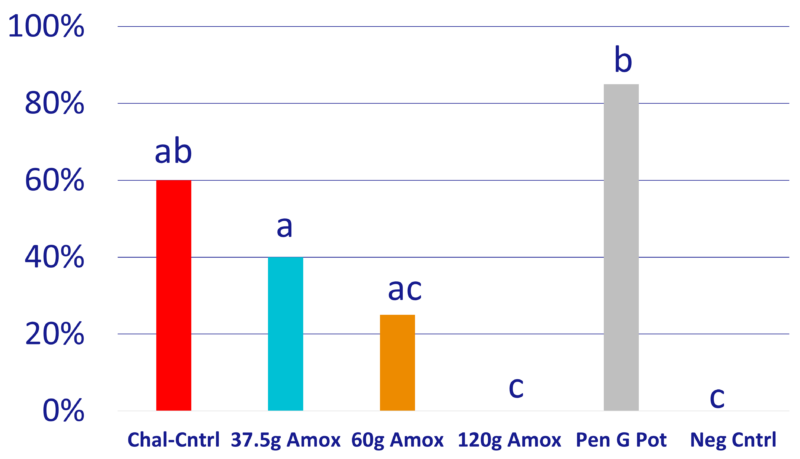
Chart 2: Number dead pigs during trial phase
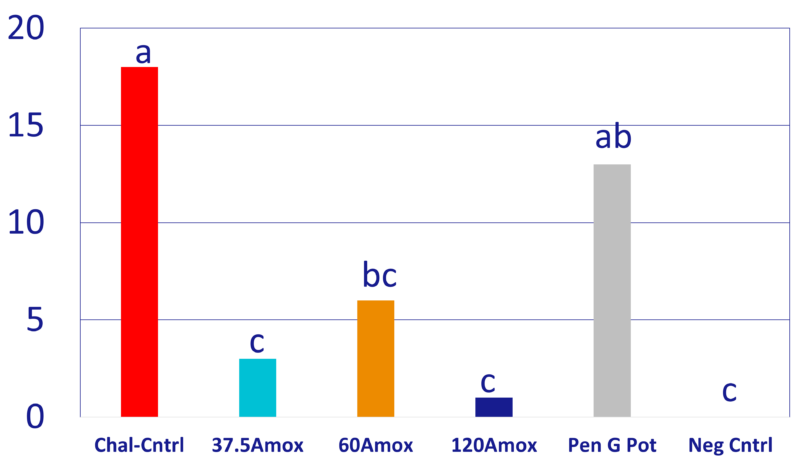
Chart 3: Mean ADG, inc. mortality, lbs
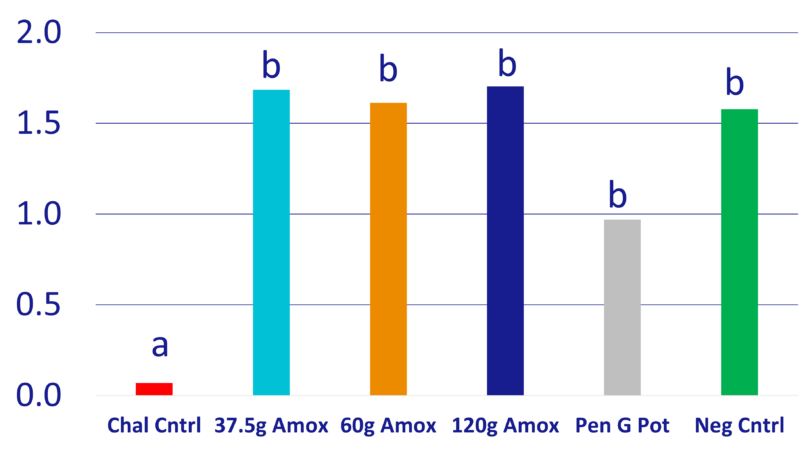
Chart 4: Trial survivors’ maximum clinical scores
Within each chart, values with different letters indicate a significant difference P < 0.05
Conclusion
Penicillin treated pigs performed similarly to challenged/non-treated pigs. All amoxicillin-treated groups outperformed the penicillin group. The highest amoxicillin dosing had similar performance to non-challenged pigs.
Strep. suis cases requiring retreatments and resulting in chronically-infected pigs are not uncommon in the field. Though amoxicillin is a time-dependent antibiotic, low dosages may be based on solubility limitations for historic formulations and these low doses may not achieve desired clinical outcomes.2 This study provides evidence that a higher dose had a better outcome. Success with elevated dosing in younger populations may allow for overall reduction in antibiotic use and cost over the pig’s lifespan, supporting responsible use.
References
1. Swine Health Information Center, Swine Disease Reporting System Report # 76. Accessed June 08, 2024. https://www.swinehealth.org/wp-content/uploads/2024/06/SDRS-report-76B.pdf.
2. Hawkins P, et al. Amoxicillin – Let’s get the dose right! Proc AASV. 2009; 223-30


