UltrAmox: compounded water-soluble amoxicillin
UltrAmox: compounded water-soluble amoxicillin
UltrAmox: compounded water-soluble amoxicillin

As the latest patent-pending technology from the leader in amoxicillin solutions, UltrAmox™ is the most stable product available, at 90% potency at 24 hours.1
The Veterinary Pharmaceutical Solutions (VPS)-patented formulation of Amoxicillin was launched in 1997, and Amoxicillin Red followed in 2009. Over two billion pigs have been treated with VPS prescription amoxicillin formulations. UltrAmox, specifically designed for livestock and poultry, is the most recent advancement in amoxicillin and was developed in 2024 for its higher potency over time.2
Product details
Available sizes: 37.5 – 300 grams
Common prescription dosage: Up to 120 grams per gallon of stock solution
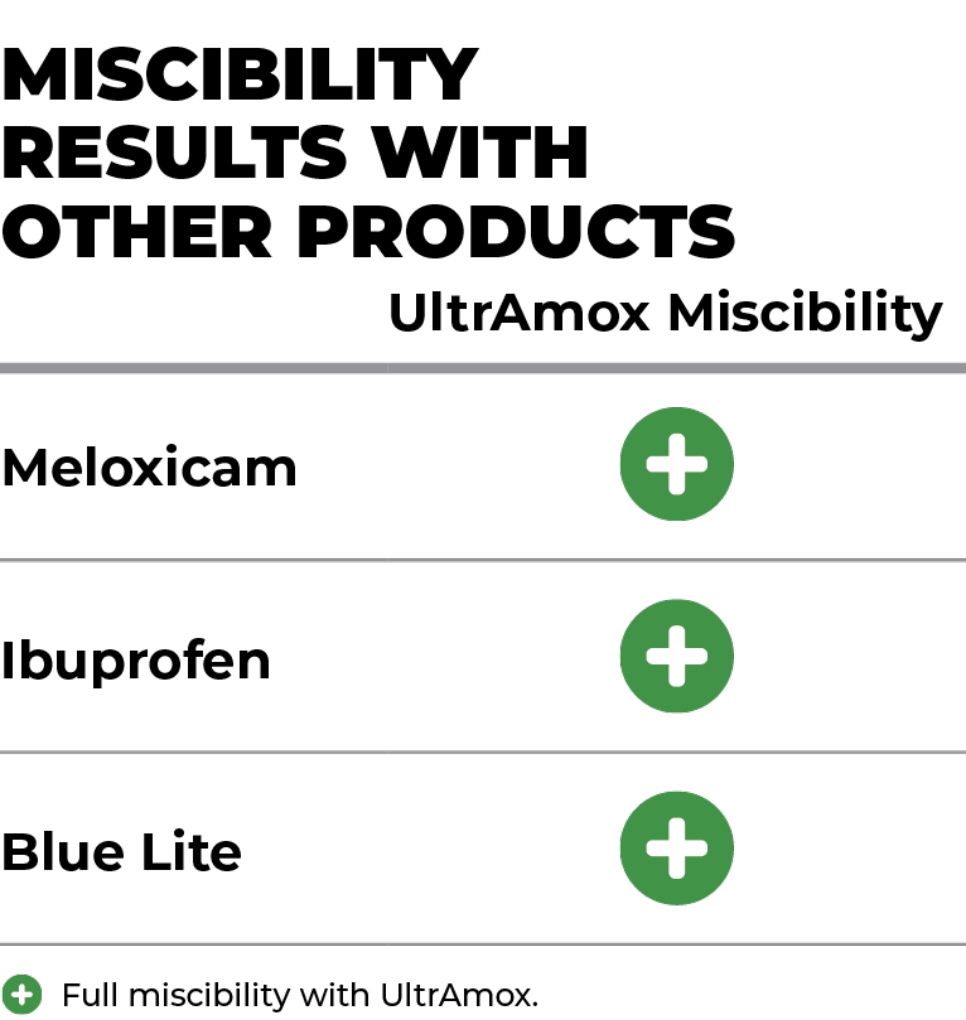
Miscibility: Meloxicam, Ibuprofen, Blue Lite
Do not mix with: Acids or bleach
Withdrawal period: 15 days
Stability: Highest stability product on the market with over 90% potency at 24 hours in multiple internal studies

Optimal Miscibility

UltrAmox best practices3-6
1 Veterinary Pharmaceutical Solutions. Data on file. Internal studies conducted on known available compounded amoxicillin products.
2 Veterinary Pharmaceutical Solutions. Data on file. Higher potency reached when handled properly.
3 Akers MJ. Formulation and Stability of Solutions. Int J Pharm Compd. 2016 Jan-Feb;20(1):41-5.
4 Gikonyo D, Gikonyo A, Luvayo D, Ponoth P. Drug expiry debate: the myth and the reality. Afr Health Sci. 2019;19(3):2737-2739
5 Binson G, Grignon C, Le Moal G, et al. Overcoming stability challenges during continuous intravenous administration of high-dose amoxicillin using portable elastomeric pumps. PLoS One. 2019;14(8):e0221391.
6 Xuexiang He, Stephen P. Mezyk, Irene Michael, Despo Fatta-Kassinos, Dionysios D. Dionysiou. Degradation kinetics and mechanism of β-lactam antibiotics by the activation of H2O2 and Na2S2O8 under UV-254nm irradiation. Journal of Hazardous Materials. 2014;279:375-383.
Disclaimer: These statements have not been evaluated by the Food and Drug Administration.




